Information about DURAN®
Beakers, Erlenmeyer flasks, round bottom flasks and test tubes, assemblies
of flasks, coolers, distillation links and other well known glass components
characterise the image of the chemical laboratory. Glass has a long tradition in
laboratories and was continuously improved to fulfill the growing demands
of chemical laboratories:
- Excellent chemical resistance
- Minimum ion transfer
- Maximum constancy of shape and volume
- Heat resistance and temperature shock resistance
- Transparency
What is glass?
under cooling. The basic components are network formers and network
modifiers. Typical forming components are SiO2, B2O3, P2O5 and depending on
certain conditions Al2O3. These components are capable of absorbing metal
oxides up to a certain proportion without losing their glassy character. This means
that the incorporated oxides are not involved in the formation of the glass
but modify certain physical properties of the structure of the glass as ”network
modifiers“.
A large number of chemical substances have the property to solidify from
the molten state into a glassy state. The formation of glass prerequisites the
existence of mixed types of bonds (covalent bonds and ionic bonds) between
the atoms or groups of atoms depending on the cooling rate. In the molten
state they show a strong tendency towards amorphous three-dimensional
networking through polimersation. Glass forms a largely amorphous ”network“
when it cools down from the molten state. The components mainly involved in
the formation of the glass are therefore described as ”network formers“. The
glass forming molecules in this network can incorporate ions that open up the
network at certain points, changing its structure and thus the properties of the
glass. Therefore they are called ”network modifiers“.
DURAN® glass
Very high chemical resistance, nearly inert behaviour, a high usage temperature,
minimal thermal expansion and the resultant high resistance to thermal shock
are the most significant properties of DURAN® glass. This optimal physical
and chemical performance makes DURAN® the ideal material for use in the
laboratory and for the manufacture of chemical apparatuses used in large
scale industrial plants. It is also widely used on an industrial scale in all other
application areas in which extreme heat resistance, resistance to thermal shock,
mechanical strength and exceptional chemical resistance are required.
DURAN® properties are specified in DIN ISO 3585. In contrast to other borosilicate
glasses, DURAN® is notable for its highly consistent, technically reproducible quality.
Chemical composition of DURAN®
| SiO2 | 81 Gew.-% |
| B2O3 | 13 Gew.-% |
| Na2O/K2O | 4 Gew.-% |
| Al2O3 | 2 Gew.-% |
DURAN® glass is highly resistant to water, acids, saline solutions, organic
substances and also halogens such as chlorine and bromine. The resistance to
alkali is also relatively good. Only hydrofluoric acid, concentrated phosphoric
acid and strong alkalis cause appreciable surface removal of the glass (glass
corrosion) at elevated temperatures. Due to the nearly inert behaviour, there
are no interactions (e.g. ion exchange) between medium and glass and any
spurious influence on experiments is thereby effectively excluded.
Hydrolytic resistance
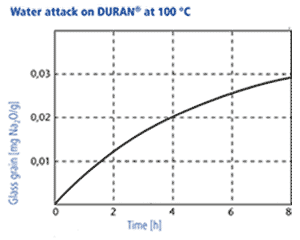
The resistance is determined with two methods, at 98 °C and at 121 °C: 1. Acc. to DIN ISO 719 DURAN® corresponds to hydrolytic resistance class 1 (of five classes). The amount of Na2O/g glass grain leached out after one hour in water at 98 °C is measured. For DURAN® the quantity of Na2O leached out is less than 3 μg/g of glass grain. 2. DURAN® also corresponds to hydrolytic resistance class 1 acc. to DIN ISO 720 (of three classes). The quantity of Na2O leached out after one hour in water at 121 °C is less than 62 μg/g of glass grain. Due to its good hydrolytic resistance DURAN® meets the requirements of the USP, JP and EP for a neutral glass according to glass type 1. Therefore it can be used in an almost unrestricted way in pharmaceutical applications and in contact with foodstuffs.
The resistance is determined with two methods, at 98 °C and at 121 °C: 1. Acc. to DIN ISO 719 DURAN® corresponds to hydrolytic resistance class 1 (of five classes). The amount of Na2O/g glass grain leached out after one hour in water at 98 °C is measured. For DURAN® the quantity of Na2O leached out is less than 3 μg/g of glass grain. 2. DURAN® also corresponds to hydrolytic resistance class 1 acc. to DIN ISO 720 (of three classes). The quantity of Na2O leached out after one hour in water at 121 °C is less than 62 μg/g of glass grain. Due to its good hydrolytic resistance DURAN® meets the requirements of the USP, JP and EP for a neutral glass according to glass type 1. Therefore it can be used in an almost unrestricted way in pharmaceutical applications and in contact with foodstuffs.
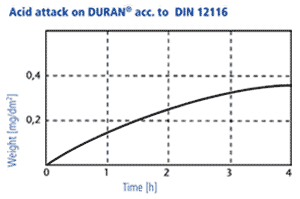
Acid resistance
Acid resistance can be determined by two methods:
1. In accordance with DIN ISO 12116
DURAN® corresponds to class 1 (of four classes). The acid removal is measured at fire finished glass surfaces, as a time dependent weight loss under the exposure of 18 % hydrochloric acid. After a boiling period of three hours this removal is only 0,3 mg/dm2.
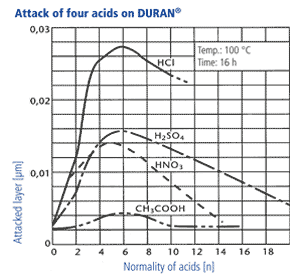
2. In accordance with DIN ISO 1776 the attacked layer thickness of the glass is examined in dependancy of the type of acid and its concentration. The results for four acids are shown in the diagram beside. The maximum attack occurs at acid ranges of 4-7 n. At higher concentrations, the reaction rate decreases significantly, so that the layer thicknesses which are attacked are only in the range of a few thousand μm after years. Thus, the mechanisms of acid attack are not relevant for the wall thicknesses of laboratory glasses used in practice.
Acid resistance can be determined by two methods:
1. In accordance with DIN ISO 12116
DURAN® corresponds to class 1 (of four classes). The acid removal is measured at fire finished glass surfaces, as a time dependent weight loss under the exposure of 18 % hydrochloric acid. After a boiling period of three hours this removal is only 0,3 mg/dm2.
2. In accordance with DIN ISO 1776 the attacked layer thickness of the glass is examined in dependancy of the type of acid and its concentration. The results for four acids are shown in the diagram beside. The maximum attack occurs at acid ranges of 4-7 n. At higher concentrations, the reaction rate decreases significantly, so that the layer thicknesses which are attacked are only in the range of a few thousand μm after years. Thus, the mechanisms of acid attack are not relevant for the wall thicknesses of laboratory glasses used in practice.
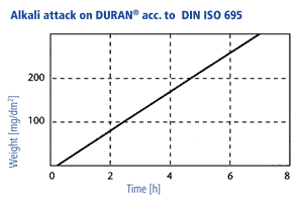
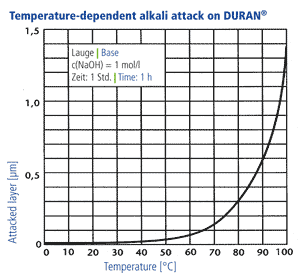
Alkali resistance
In accordance with DIN ISO 695 DURAN® corresponds to alkali resistance class 2 (of three classes). The surface erosion after three hours boiling in a mixture of equal volume fractions of sodium hydroxide solution (concentration 1 mol/l) and sodium carbonate solution (concentration 0,5 mol/l) is only 134 mg/100 cm2. The surface removal through alkali ist directly proportional to time. A visible attack on the glass surface takes place only at temperatures above 60 °C, at lower temperatures the reaction rates are so low that hardly any reduction of the wall thickness takes place over a period of years. Long-term tests have shown that the use of NaOH with a concentration of 1 mol/l at an operating temperature of 50 °C produces a glass surface removal of 1 mm after 25 years in a continuous flow through a DURAN® glass pipeline.
Temperature resistance when heated and thermal shock resistance
The maximum short-term operating temperature of DURAN is 500 °C, the recommended permissible temperature of DURAN is 350 °C. This temperature may be exceeded for a short period. Above a temperature of 560 °C DURAN begins to soften and at 820 °C it changes to the liquid state. As it has a very low coefficient of linear expansion (a = 3.3 x 10-6K-1), a feature of DURAN ® is its high thermal shock resistance (up to DT = 100 K). For a temperature change of 1 K, the glass hanges by only 3.3 x 10-6 relative length units, resulting in low levels of mechanical strain where a thermal gradient exists. The thermal shock resistance is depending on the wall thickness and geometry of the products.
In accordance with DIN ISO 695 DURAN® corresponds to alkali resistance class 2 (of three classes). The surface erosion after three hours boiling in a mixture of equal volume fractions of sodium hydroxide solution (concentration 1 mol/l) and sodium carbonate solution (concentration 0,5 mol/l) is only 134 mg/100 cm2. The surface removal through alkali ist directly proportional to time. A visible attack on the glass surface takes place only at temperatures above 60 °C, at lower temperatures the reaction rates are so low that hardly any reduction of the wall thickness takes place over a period of years. Long-term tests have shown that the use of NaOH with a concentration of 1 mol/l at an operating temperature of 50 °C produces a glass surface removal of 1 mm after 25 years in a continuous flow through a DURAN® glass pipeline.
Temperature resistance when heated and thermal shock resistance
The maximum short-term operating temperature of DURAN is 500 °C, the recommended permissible temperature of DURAN is 350 °C. This temperature may be exceeded for a short period. Above a temperature of 560 °C DURAN begins to soften and at 820 °C it changes to the liquid state. As it has a very low coefficient of linear expansion (a = 3.3 x 10-6K-1), a feature of DURAN ® is its high thermal shock resistance (up to DT = 100 K). For a temperature change of 1 K, the glass hanges by only 3.3 x 10-6 relative length units, resulting in low levels of mechanical strain where a thermal gradient exists. The thermal shock resistance is depending on the wall thickness and geometry of the products.
Temperature resistance at low temperatures
DURAN® can be cooled down to the maximum possible negative temperature and is therefore suitable for use with liquid nitrogen (approx. -196 °C). During such freezing you have to observe the expansion of the content. In general DURAN® products are recommended for use down to -70 °C. Besides the geometry of the products you also have to pay attention to the property of the used components. During cooling and thawing ensure that the temperature difference does not exceed 100 K. In practice, stepwise cooling and heating are recommended.
Use in the microwave DURAN® laboratory glassware is suitable for use in microwaves.
Physical properties of common technical glasses
DURAN® can be cooled down to the maximum possible negative temperature and is therefore suitable for use with liquid nitrogen (approx. -196 °C). During such freezing you have to observe the expansion of the content. In general DURAN® products are recommended for use down to -70 °C. Besides the geometry of the products you also have to pay attention to the property of the used components. During cooling and thawing ensure that the temperature difference does not exceed 100 K. In practice, stepwise cooling and heating are recommended.
Use in the microwave DURAN® laboratory glassware is suitable for use in microwaves.
Physical properties of common technical glasses
| Description | Linear expansion coefficient  (20 °C / 300 °C) |
Transformation temperature (°C) |
Density (g/cm³) |
| DURAN® | 3,3 | 525 | 2,23 |
| Soda lime glass | 9,1 | 525 | 2,5 |
| SBW | 6,5 | 555 | 2,45 |
Optical properties
DURAN® is transparent and colourless. In the spectral range from about 310 to
2200 nm the absorption of DURAN® is negligibly low. Fairly large layer thicknesses (axial view through pipes) appear slightly yellow/greenish. Amber-coloured DURAN® products are suited to use with light-sensitive substances. This results in strong absorption in the short-wave region up to approx. 500 nm. In photochemical processes the light transmission of DURAN® in the ultraviolet range is of particular importance. The degree of light transmission of DURAN® in the ultraviolet range indicates that photochemical reactions can be carried out, for example chlorination and sulfochlorination. The chlorine molecule absorbs light in the range from 280 to 400 nm and thus serves as a transmitter of the radiation energy.
DURAN® is transparent and colourless. In the spectral range from about 310 to
2200 nm the absorption of DURAN® is negligibly low. Fairly large layer thicknesses (axial view through pipes) appear slightly yellow/greenish. Amber-coloured DURAN® products are suited to use with light-sensitive substances. This results in strong absorption in the short-wave region up to approx. 500 nm. In photochemical processes the light transmission of DURAN® in the ultraviolet range is of particular importance. The degree of light transmission of DURAN® in the ultraviolet range indicates that photochemical reactions can be carried out, for example chlorination and sulfochlorination. The chlorine molecule absorbs light in the range from 280 to 400 nm and thus serves as a transmitter of the radiation energy.
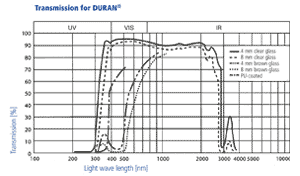
Amber colouring of DURAN® laboratory glassware
Amber colouring enables storage of light sensitive substances in DURAN® products. To colour DURAN® glassware, it is sprayed using an innovative process with a special medium-diffusion ink solely on the outside of the clear glass. On cooling, the ambering is very uniform, resistant to chemicals and cleaning in a dishwasher. The proven DURAN® properties within the bottle remain unaffected; there is no contact or interaction between contents and
amber coating.
Conformity with standards and guidelines
Besides the international standard DIN ISO 3585, in which the properties of borosilicate glass 3.3 are defined, DURAN® laboratory glassware corresponds to the current standards for glass laboratory apparatuses. The relevant DIN/ ISO standards are given on the product pages of this catalogue. DURAN® is a neutral glass of high hydrolytic resistance and thus belongs to glass type I in accordance with the European pharmacopeia (EP, chapter 3.2.1), the Japanese pharmacopeia (JP, chapter 7.01) and the United States pharmacopeia (USP, section: 660) and National Formulary.
Cleaning of laboratory glassware
Laboratory glass apparatuses can be washed by hand in a soaking bath or by machine in a lab washer. As contamination during the delivery of the laboratory glassware cannot be totally ruled out, we recommend washing laboratory glassware before it is used for the first time. To care properly for laboratory glassware, it should be washed at low temperature, on a short cycle and with low alkalinity immediately after use. Laboratory apparatuses that have come into contact with infectious substances or microorganisms should be treated in accordance with the current guidelines. Dependent on the substance, autoclaving (e.g. to kill microorganisms) may be necessary prior to cleaning.
1. Manual cleaning
The generally recognized method is to wipe and rub the glass with a cloth or sponge soaked in cleaning solution. Abrasive cleaners and abrasive sponges should not be used on laboratory glassware as these can damage the surface of the glass. Surface damage can affect the glass properties and limit further
use of the product. In a soaking bath the laboratory glass should generally be left in the cleaning solution for 20 to 30 minutes at room temperature, then rinsed with tap water followed by distilled water. Only in case of persistent soiling a prolonged soaking time and higher temperature should be used. Laboratory glassware should not be soaked for long periods in strongly alkaline media at more than 70 °C since this can have an adverse effect on the ceramic printing and may cause glass corrosion. Also strong mechanical stress should be avoided.
2. Machine cleaning
The machine-based cleaning of laboratory glassware in laboratory dishwashers is a milder treatment than cleaning in a dipping bath, since the glass comes only in contact with cleaning solutions during a relatively short period of time. This conserves glass surfaces and ceramic prints. Use the customary cleaning solutions for laboratory dishwashers.
3. Disinfection of laboratory glassware
Laboratory glass instruments can be disinfected. With manual cleaning use a disinfectant cleaning solution. With machine cleaning use physical thermal processes (10 minutes residence time at 93 °C according to BGA) or chemothermal processes. After that laboratory instruments can be autoclaved.
4. Autoclaving of laboratory glass
According to DIN 58900, part 1 and DIN 58946, part 1/2, 1987, hot air sterilisation is the „killing resp. irreversible diasabling of all augmentable microorganisms“ under the influence of „saturated steam of at least 120 °C
and 2 bar“. As minimum residence time (time to kill + excess time) is considered
te = 20 minutes at 121 °C. A raised vapour temperature of 121 °C is only possible with a raised pressure of 2 bars. Vessels must only be hot air sterilised with open closures, to avoid additional pressure build-up resulting in breakage.
Notes concerning sterilisation
Important safety tips for users
Disposal
DURAN® laboratory glass should under no circumstances be disposed of in the domestic glass recycling system. Because of its high melting point and different chemistry, DURAN® is not compatible for the recycling with other glass types (soda-lime glass). The correct way to dispose of it, is in principle, to include it with general household waste (residual waste) in accordance with the relevant guidelines, provided that the glass is quite free of any harmful contamination.
Amber colouring enables storage of light sensitive substances in DURAN® products. To colour DURAN® glassware, it is sprayed using an innovative process with a special medium-diffusion ink solely on the outside of the clear glass. On cooling, the ambering is very uniform, resistant to chemicals and cleaning in a dishwasher. The proven DURAN® properties within the bottle remain unaffected; there is no contact or interaction between contents and
amber coating.
Conformity with standards and guidelines
Besides the international standard DIN ISO 3585, in which the properties of borosilicate glass 3.3 are defined, DURAN® laboratory glassware corresponds to the current standards for glass laboratory apparatuses. The relevant DIN/ ISO standards are given on the product pages of this catalogue. DURAN® is a neutral glass of high hydrolytic resistance and thus belongs to glass type I in accordance with the European pharmacopeia (EP, chapter 3.2.1), the Japanese pharmacopeia (JP, chapter 7.01) and the United States pharmacopeia (USP, section: 660) and National Formulary.
Cleaning of laboratory glassware
Laboratory glass apparatuses can be washed by hand in a soaking bath or by machine in a lab washer. As contamination during the delivery of the laboratory glassware cannot be totally ruled out, we recommend washing laboratory glassware before it is used for the first time. To care properly for laboratory glassware, it should be washed at low temperature, on a short cycle and with low alkalinity immediately after use. Laboratory apparatuses that have come into contact with infectious substances or microorganisms should be treated in accordance with the current guidelines. Dependent on the substance, autoclaving (e.g. to kill microorganisms) may be necessary prior to cleaning.
1. Manual cleaning
The generally recognized method is to wipe and rub the glass with a cloth or sponge soaked in cleaning solution. Abrasive cleaners and abrasive sponges should not be used on laboratory glassware as these can damage the surface of the glass. Surface damage can affect the glass properties and limit further
use of the product. In a soaking bath the laboratory glass should generally be left in the cleaning solution for 20 to 30 minutes at room temperature, then rinsed with tap water followed by distilled water. Only in case of persistent soiling a prolonged soaking time and higher temperature should be used. Laboratory glassware should not be soaked for long periods in strongly alkaline media at more than 70 °C since this can have an adverse effect on the ceramic printing and may cause glass corrosion. Also strong mechanical stress should be avoided.
2. Machine cleaning
The machine-based cleaning of laboratory glassware in laboratory dishwashers is a milder treatment than cleaning in a dipping bath, since the glass comes only in contact with cleaning solutions during a relatively short period of time. This conserves glass surfaces and ceramic prints. Use the customary cleaning solutions for laboratory dishwashers.
3. Disinfection of laboratory glassware
Laboratory glass instruments can be disinfected. With manual cleaning use a disinfectant cleaning solution. With machine cleaning use physical thermal processes (10 minutes residence time at 93 °C according to BGA) or chemothermal processes. After that laboratory instruments can be autoclaved.
4. Autoclaving of laboratory glass
According to DIN 58900, part 1 and DIN 58946, part 1/2, 1987, hot air sterilisation is the „killing resp. irreversible diasabling of all augmentable microorganisms“ under the influence of „saturated steam of at least 120 °C
and 2 bar“. As minimum residence time (time to kill + excess time) is considered
te = 20 minutes at 121 °C. A raised vapour temperature of 121 °C is only possible with a raised pressure of 2 bars. Vessels must only be hot air sterilised with open closures, to avoid additional pressure build-up resulting in breakage.
Notes concerning sterilisation
- Contaminated laboratory instruments must be cleaned before sterilisation.
- Dirt particles bake and enclose microorganisms, so that they are protected by the dirt particles and cannot be effectively killed. Chemicals embedded in dirt particles can attack the surface because of the high temperatures during the sterilisation process.
- To avoid overpressure, the vessels should be always kept open.
- Effective sterilisation is only possible with saturated vapour which can reach unhindered any part of the contaminated vessel.
Important safety tips for users
- For safety reasons, before DURAN® laboratory glassware is used, it should be checked to ensure that it is suitable for the intended purpose.
- Defective laboratory glassware represents a risk ( e.g. risk of cuts, burns, infection) that should not be underestimated. If appropriate repairs cannot be carried out or cannot be justified for economic reasons, it must be disposed of in the proper manner.
- Repairs must only be carried out by skilled competent glassworkers. Poorly repaired glassware can fail without warning and represents a significant hazard.
- Subject DURAN® glassware to sudden temperature changes only within the recommended limit for thermal shock resistance (T = 100 K).
- Apparatuses have to be assembled stable and stressless.
- Pressurized or evacuated glass apparatus must never be touched to avoid surface damage.
- To avoid tensions in the glass, heat up evacuated or pressurized glassware evenly and never in an open flame.
- Previous to evacuating or pressurizing glass instruments, a visual check is required to secure proper conditions.
- Glassware must never be subjected to sudden pressure changes, like sudden venting.
Disposal
DURAN® laboratory glass should under no circumstances be disposed of in the domestic glass recycling system. Because of its high melting point and different chemistry, DURAN® is not compatible for the recycling with other glass types (soda-lime glass). The correct way to dispose of it, is in principle, to include it with general household waste (residual waste) in accordance with the relevant guidelines, provided that the glass is quite free of any harmful contamination.



